Excellent Customer Service

Best Prices Around


Accredited by UK Vets Association
Delivered Direct To Your Door
The medicine can only be obtained with a prescription and is not available to purchase from a pet shop. As this medication is a Schedule Three Drug, we require the original prescription posting to us.
Tralieve Chewable Tablets for Dogs are for the reduction of acute and chronic mild soft tissue and musculoskeletal pain.
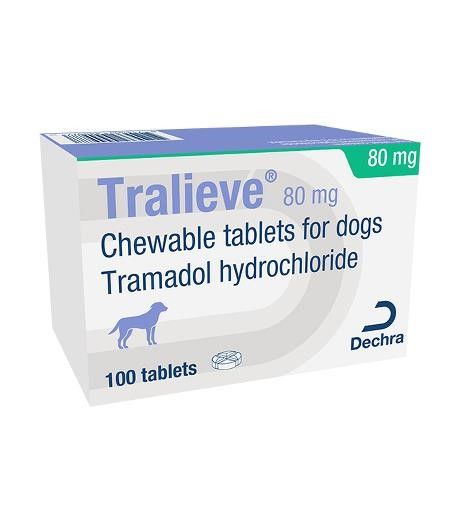
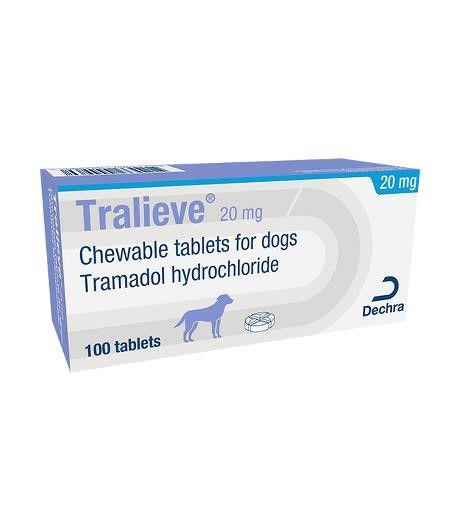
Tralieve® Chewable Tablets for Dogs
Qualitative and quantitative composition
Tralieve 20 mg chewable tablets for dogs
1 tablet contains: Active substance:
Tramadol hydrochloride 20 mg
(equivalent to 17.6 mg tramadol)
Tralieve 80 mg chewable tablets for dogs
1 tablet contains: Active substance:
Tramadol hydrochloride 80 mg
(equivalent to 70.3 mg tramadol)
Pharmaceutical form
Chewable tablet.
20 mg tablet: Light brown with brown spots, round and convex flavoured 7 mm tablet with a cross-shaped break line on one side.
80 mg tablet: Light brown with brown spots, round and convex flavoured 11 mm tablet with a cross-shaped break line on one side.
Tablets can be divided into 2 or 4 equal parts.
Clinical particulars
Target species
Dogs.
Indications for use
For the reduction of acute and chronic mild soft tissue and musculoskeletal pain.
Contraindications
Do not administer in conjunction with tricyclic antidepressants, monoamine oxidase inhibitors and serotonin reuptake inhibitors.
Do not use in cases of hypersensitivity to tramadol or to any of the excipients.
Do not use in animals with epilepsy.
Special warnings for each target species
The analgesic effects of tramadol hydrochloride may be variable. This is thought to be due to individual differences in the metabolism of the drug to the primary active metabolite O-desmethyltramadol. In some dogs (non-responders) this may result in the product failing to provide analgesia. For chronic pain, multimodal analgesia should be considered. Dogs should be monitored regularly by a veterinarian to ensure adequate pain relief. In case of recurrence of pain or insufficient analgesia the analgesic protocol may need to be reconsidered.
Special precautions for use in animals
Use with caution in dogs with renal or hepatic impairment. In dogs with hepatic impairment the metabolism of tramadol to the active metabolites may be decreased which may reduce the efficacy of the product. One of the active metabolites of tramadol is renally excreted and therefore in dogs with renal impairment the dosing regimen used may need to be adjusted. Renal and hepatic function should be monitored when using this product. Cessation of long-term analgesic therapy should be done gradually whenever possible.
Special precautions to be taken by the person administering the veterinary medicinal product to animals
Tramadol may cause sedation, nausea and dizziness after accidental ingestion, especially by children. To avoid accidental ingestion, particularly by a child, unused tablet parts should be returned to the open blister space and inserted back into the carton and kept in a safe place out of the sight and reach of children as they pose a health risk to small children due to accidental ingestion. In case of accidental ingestion, particularly by children, seek medical advice and show the package leaflet or the label to the physician. In case of accidental ingestion by adults: DO NOT DRIVE as sedation may occur.
People with known hypersensitivity to tramadol or any of the excipients should avoid contact with the veterinary medicinal product.
Wash hands after use.
Adverse reactions
Mild sedation and drowsiness may commonly occur, especially when higher doses are given.
Nausea and vomiting have uncommonly been observed in dogs after administration of tramadol.
In rare cases hypersensitivity can occur. In cases of hypersensitivity reactions the treatment should be discontinued.
In very rare cases tramadol may induce convulsions in dogs with a low seizure threshold.
The frequency of adverse reactions is defined using the following convention:
- very common (more than 1 in 10 animals treated displaying adverse reaction(s))
- common (more than 1 but less than 10 animals in 100 animals treated)
- uncommon (more than 1 but less than 10 animals in 1,000 animals treated)
- rare (more than 1 but less than 10 animals in 10,000 animals treated)
- very rare (less than 1 animal in 10,000 animals treated, including isolated reports)
Use during pregnancy and lactation
Pregnancy:
Laboratory studies in mice and/or rats and rabbits have not produced any evidence of teratogenic, foetotoxic, maternotoxic effects. Use only according to the benefit-risk assessment by the responsible veterinarian.
Lactation:
Laboratory studies in mice and/or rats and rabbits have not produced any evidence of adverse effects in the peri- and postnatal development of offspring. Use only according to the benefit-risk assessment by the responsible veterinarian.
Fertility:
In laboratory studies in mice and/or rats and rabbits, the use of tramadol at therapeutic doses did not adversely affect reproductive performance and fertility in males and females. Use only according to the benefit-risk assessment by the responsible veterinarian.
Interactions
Concomitant administration of the product with central nervous system depressants, may potentiate the CNS and respiratory depressant effects. Tramadol can increase the effect of drugs that lower the seizure threshold.
Drugs that inhibit (e.g. cimetidine and erythromycin) or induce (e.g. carbamazepine) CYP450 mediated metabolism may have an effect on the analgesic effect of tramadol. The clinical relevance of these interactions has not been studied in dogs.
The combination with mixed agonist/antagonists (e.g. buprenorphine, butorphanol) and tramadol is not advisable, because the analgesic effect of a pure agonist may be theoretically reduced in such circumstances.
See also Contraindications.
Amounts to be administered and administration route
For oral administration.
The recommended dose is 2-4 mg tramadol hydrochloride per kg body weight every 8 hours or as needed based on the intensity of pain.
Minimum dosing interval is 6 hours. The recommended maximum daily dose is 16 mg/kg. As the individual response to tramadol is variable and depends partly on the dosage, the age of the patient, individual differences in pain sensitivity and general condition, the optimal dosing regimen should be individually tailored using the above dose and re-treatment interval ranges. The dog should be examined regularly by a veterinarian to assess if additional analgesia is subsequently required. Additional analgesia can be administered by increasing the tramadol dose until the maximum daily dose is reached, and/or by following a multimodal analgesic approach with the addition of other suitable analgesics.
The most appropriate tablet strengths should be used in order to minimise divided tablets to be kept until the next dosing.
Please note that this dosing table is intended as a guide for dispensing the product at the high end of the dose range: 4 mg/kg body weight. It states the number of tablets required to administer 4 mg tramadol hydrochloride per kg body weight.
Tablets can be divided into 2 or 4 equal parts to ensure accurate dosing. Place the tablet on a flat surface, with its scored side facing up and the convex (rounded) side facing the surface.
2 equal parts: press down with your thumbs on both sides of the tablet.
4 equal parts: press down with your thumb in the middle of the tablet.
Overdose
In cases of intoxication with tramadol symptoms similar to those observed with other centrally acting analgesics (opioids) are likely to occur. These include in particular miosis, vomiting, cardiovascular collapse, consciousness disorders up to coma, convulsions and respiratory depression up to respiratory arrest.
General emergency measures: Maintain a patent airway, support cardiac and respiratory function depending on the symptoms. Inducing vomiting in order to empty the stomach is suitable unless the affected animal is showing reduced consciousness, in which case gastric lavage may be considered. The antidote for respiratory depression is naloxone. However, naloxone may not be useful in all cases of tramadol overdose as it may only partially reverse some of the other effects of tramadol. In case of seizures, administer diazepam.
The medicine can only be obtained with a prescription and is not available to purchase from a pet shop. As this medication is a Schedule Three Drug, we require the original prescription posting to us.
Legal Category: POM-V CD3
To find details on all veterinary medicinal products currently authorised in the UK, please see the VMD's Product Information Database.
IMPORTANT LEGAL INFORMATION: this is a Veterinary Prescription Only Medicine (POM-V), which can only be prescribed by a veterinary surgeon, but may be dispensed by another veterinary surgeon or pharmacist.
DESCRIPTION
The medicine can only be obtained with a prescription and is not available to purchase from a pet shop. As this medication is a Schedule Three Drug, we require the original prescription posting to us.
Tralieve Chewable Tablets for Dogs are for the reduction of acute and chronic mild soft tissue and musculoskeletal pain.
Tralieve® Chewable Tablets for Dogs
Qualitative and quantitative composition
Tralieve 20 mg chewable tablets for dogs
1 tablet contains: Active substance:
Tramadol hydrochloride 20 mg
(equivalent to 17.6 mg tramadol)
Tralieve 80 mg chewable tablets for dogs
1 tablet contains: Active substance:
Tramadol hydrochloride 80 mg
(equivalent to 70.3 mg tramadol)
Pharmaceutical form
Chewable tablet.
20 mg tablet: Light brown with brown spots, round and convex flavoured 7 mm tablet with a cross-shaped break line on one side.
80 mg tablet: Light brown with brown spots, round and convex flavoured 11 mm tablet with a cross-shaped break line on one side.
Tablets can be divided into 2 or 4 equal parts.
Clinical particulars
Target species
Dogs.
Indications for use
For the reduction of acute and chronic mild soft tissue and musculoskeletal pain.
Contraindications
Do not administer in conjunction with tricyclic antidepressants, monoamine oxidase inhibitors and serotonin reuptake inhibitors.
Do not use in cases of hypersensitivity to tramadol or to any of the excipients.
Do not use in animals with epilepsy.
Special warnings for each target species
The analgesic effects of tramadol hydrochloride may be variable. This is thought to be due to individual differences in the metabolism of the drug to the primary active metabolite O-desmethyltramadol. In some dogs (non-responders) this may result in the product failing to provide analgesia. For chronic pain, multimodal analgesia should be considered. Dogs should be monitored regularly by a veterinarian to ensure adequate pain relief. In case of recurrence of pain or insufficient analgesia the analgesic protocol may need to be reconsidered.
Special precautions for use in animals
Use with caution in dogs with renal or hepatic impairment. In dogs with hepatic impairment the metabolism of tramadol to the active metabolites may be decreased which may reduce the efficacy of the product. One of the active metabolites of tramadol is renally excreted and therefore in dogs with renal impairment the dosing regimen used may need to be adjusted. Renal and hepatic function should be monitored when using this product. Cessation of long-term analgesic therapy should be done gradually whenever possible.
Special precautions to be taken by the person administering the veterinary medicinal product to animals
Tramadol may cause sedation, nausea and dizziness after accidental ingestion, especially by children. To avoid accidental ingestion, particularly by a child, unused tablet parts should be returned to the open blister space and inserted back into the carton and kept in a safe place out of the sight and reach of children as they pose a health risk to small children due to accidental ingestion. In case of accidental ingestion, particularly by children, seek medical advice and show the package leaflet or the label to the physician. In case of accidental ingestion by adults: DO NOT DRIVE as sedation may occur.
People with known hypersensitivity to tramadol or any of the excipients should avoid contact with the veterinary medicinal product.
Wash hands after use.
Adverse reactions
Mild sedation and drowsiness may commonly occur, especially when higher doses are given.
Nausea and vomiting have uncommonly been observed in dogs after administration of tramadol.
In rare cases hypersensitivity can occur. In cases of hypersensitivity reactions the treatment should be discontinued.
In very rare cases tramadol may induce convulsions in dogs with a low seizure threshold.
The frequency of adverse reactions is defined using the following convention:
- very common (more than 1 in 10 animals treated displaying adverse reaction(s))
- common (more than 1 but less than 10 animals in 100 animals treated)
- uncommon (more than 1 but less than 10 animals in 1,000 animals treated)
- rare (more than 1 but less than 10 animals in 10,000 animals treated)
- very rare (less than 1 animal in 10,000 animals treated, including isolated reports)
Use during pregnancy and lactation
Pregnancy:
Laboratory studies in mice and/or rats and rabbits have not produced any evidence of teratogenic, foetotoxic, maternotoxic effects. Use only according to the benefit-risk assessment by the responsible veterinarian.
Lactation:
Laboratory studies in mice and/or rats and rabbits have not produced any evidence of adverse effects in the peri- and postnatal development of offspring. Use only according to the benefit-risk assessment by the responsible veterinarian.
Fertility:
In laboratory studies in mice and/or rats and rabbits, the use of tramadol at therapeutic doses did not adversely affect reproductive performance and fertility in males and females. Use only according to the benefit-risk assessment by the responsible veterinarian.
Interactions
Concomitant administration of the product with central nervous system depressants, may potentiate the CNS and respiratory depressant effects. Tramadol can increase the effect of drugs that lower the seizure threshold.
Drugs that inhibit (e.g. cimetidine and erythromycin) or induce (e.g. carbamazepine) CYP450 mediated metabolism may have an effect on the analgesic effect of tramadol. The clinical relevance of these interactions has not been studied in dogs.
The combination with mixed agonist/antagonists (e.g. buprenorphine, butorphanol) and tramadol is not advisable, because the analgesic effect of a pure agonist may be theoretically reduced in such circumstances.
See also Contraindications.
Amounts to be administered and administration route
For oral administration.
The recommended dose is 2-4 mg tramadol hydrochloride per kg body weight every 8 hours or as needed based on the intensity of pain.
Minimum dosing interval is 6 hours. The recommended maximum daily dose is 16 mg/kg. As the individual response to tramadol is variable and depends partly on the dosage, the age of the patient, individual differences in pain sensitivity and general condition, the optimal dosing regimen should be individually tailored using the above dose and re-treatment interval ranges. The dog should be examined regularly by a veterinarian to assess if additional analgesia is subsequently required. Additional analgesia can be administered by increasing the tramadol dose until the maximum daily dose is reached, and/or by following a multimodal analgesic approach with the addition of other suitable analgesics.
The most appropriate tablet strengths should be used in order to minimise divided tablets to be kept until the next dosing.
Please note that this dosing table is intended as a guide for dispensing the product at the high end of the dose range: 4 mg/kg body weight. It states the number of tablets required to administer 4 mg tramadol hydrochloride per kg body weight.
Tablets can be divided into 2 or 4 equal parts to ensure accurate dosing. Place the tablet on a flat surface, with its scored side facing up and the convex (rounded) side facing the surface.
2 equal parts: press down with your thumbs on both sides of the tablet.
4 equal parts: press down with your thumb in the middle of the tablet.
Overdose
In cases of intoxication with tramadol symptoms similar to those observed with other centrally acting analgesics (opioids) are likely to occur. These include in particular miosis, vomiting, cardiovascular collapse, consciousness disorders up to coma, convulsions and respiratory depression up to respiratory arrest.
General emergency measures: Maintain a patent airway, support cardiac and respiratory function depending on the symptoms. Inducing vomiting in order to empty the stomach is suitable unless the affected animal is showing reduced consciousness, in which case gastric lavage may be considered. The antidote for respiratory depression is naloxone. However, naloxone may not be useful in all cases of tramadol overdose as it may only partially reverse some of the other effects of tramadol. In case of seizures, administer diazepam.
The medicine can only be obtained with a prescription and is not available to purchase from a pet shop. As this medication is a Schedule Three Drug, we require the original prescription posting to us.
Legal Category: POM-V CD3
To find details on all veterinary medicinal products currently authorised in the UK, please see the VMD's Product Information Database.
IMPORTANT LEGAL INFORMATION: this is a Veterinary Prescription Only Medicine (POM-V), which can only be prescribed by a veterinary surgeon, but may be dispensed by another veterinary surgeon or pharmacist.