Excellent Customer Service

Best Price Around


Accredited by UK Vets Association
Delivered Direct To Your Door
This product is sourced in the United Kingdom and is intended for use in the United Kindgom only.
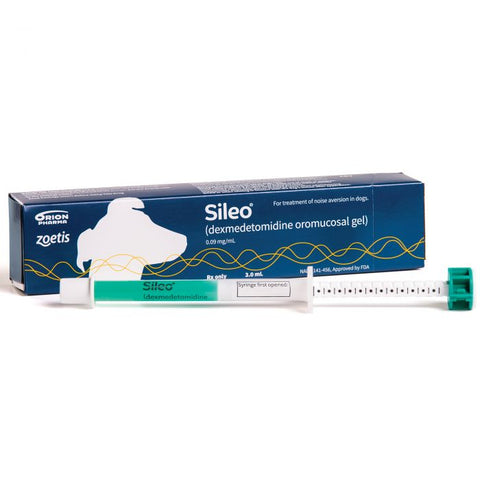
SILEO (dexmedetomidine oromucosal gel) is an oromucosal gel formulation of dexmedetomidine hydrochloride provided in a 3 mL syringe at a concentration of 0.1 mg/mL. Using the syringe, SILEO is easily administered between the cheek and gum, allowing for transmucosal absorption. Because SILEO is formulated to provide a low dose of dexmedetomidine, the dog remains calm, yet fully functional, and is able to interact normally with the family.
Sileo® 0.1 mg/ml oromucosal gel for dogs
Species: Dogs Therapeutic indication: Pharmaceuticals: Neurological preparations: Others Active ingredient: Dexmedetomidine Product:Sileo® 0.1 mg/ml oromucosal gel for dogs Product index: Sileo
Presentation
Sileo is a translucent, green oromucosal gel, with each ml containing 0.1 mg dexmedetomidine hydrochloride (equivalent to 0.09 mg dexmedetomidine).
Uses
Indicated for the alleviation of acute anxiety and fear associated with noise in dogs.
Dosage and administration
Oromucosal use. The product should be administered onto the oral mucosa between the dog’s cheek and gum at a dose of 125 micrograms/m2. The Sileo oral syringe is capable of delivering the product in 0.25 ml increments. Each increment is shown as one dot on the plunger. The dosing table provides the number of dots to be administered corresponding to the dog’s bodyweight.  The following dosing table provides the dose volume (in dots) to be administered for the corresponding bodyweight. If the dose for the dog is more than 6 dots (1.5 ml), half of the dose should be administered to the oral mucosa on one side of the dog’s mouth and the other half of the dose onto the other side. Do not exceed the recommended dose.
The following dosing table provides the dose volume (in dots) to be administered for the corresponding bodyweight. If the dose for the dog is more than 6 dots (1.5 ml), half of the dose should be administered to the oral mucosa on one side of the dog’s mouth and the other half of the dose onto the other side. Do not exceed the recommended dose.
|
Bodyweight of dog (kg) |
Number of dots |
|
2.0 - 5.5 |
1 ° |
|
5.6 - 12 |
2 ° ° |
|
12.1 - 20 |
3 ° ° ° |
|
20.1 - 29 |
4 ° ° ° ° |
|
29.1 - 39 |
5 ° ° ° ° ° |
|
39.1 - 50 |
6 ° ° ° ° ° ° |
|
50.1 - 62.5 |
7 ° ° ° ° ° ° ° |
|
62.6 - 75.5 |
8 ° ° ° ° ° ° ° ° |
|
75.6 - 89 |
9 ° ° ° ° ° ° ° ° ° |
|
89.1 - 100 |
10 ° ° ° ° ° ° ° ° ° ° |
The first dose should be given as soon as the dog shows the first signs of anxiety, or when the owner detects a typical stimulus (e.g. sound of fireworks or thunder) for eliciting anxiety or fear in the respective dog. Typical signs of anxiety and fear are panting, trembling, pacing (frequent change of place, running around, restlessness), seeking people (clinging, hiding behind, pawing, following), hiding (under furniture, in dark rooms), trying to escape, freezing (absence of movements), refusing to eat food or treats, inappropriate urination, inappropriate defecation, salivation, etc. If the fear eliciting event continues and the dog shows signs of anxiety and fear again, re-dosing can be done when 2 hours has passed from the previous dose. The product can be dosed up to 5 times during each event.
Instructions for dosing the gel:
Dosing should be performed by an adult.
New oral syringe set up before first dosing:
|
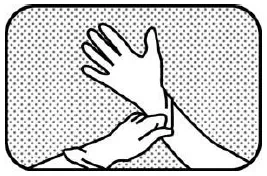
1. WEAR GLOVES Wear impermeable disposable gloves when handling the veterinary medicinal product and handling the oral gel. |
|
|
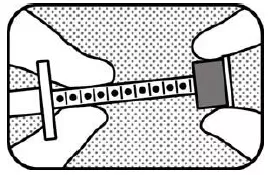
2. HOLD PLUNGER Hold the oral syringe so that you can see the dot markings on the oral syringe plunger. Hold the plunger with your left hand. |
|
|
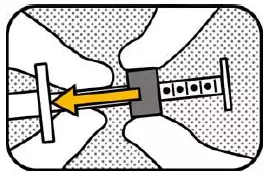
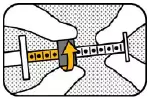
3. UNLOCK Hold the plunger with your left hand and unlock the green ring-stop by turning it towards you until it is able to slide freely. |
|
|
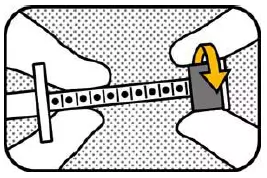
4. MOVE RING Move the ring-stop to the opposite end of the plunger. |
|
|
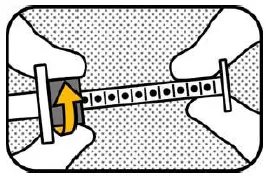
5. LOCK Hold the plunger with your right hand and lock the ring-stop by turning it away from you. |
Dose selection and dosing:
|
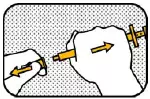
6. UNLOCK Hold the plunger with your right hand and unlock the ring-stop by turning it towards you. Do not pull the plunger! |
|
|
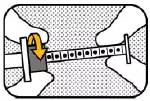
7. MOVE RING Move the ring-stop towards the other end of the plunger for choosing the correct dose based on your veterinarian's prescription. |
|
|
8. SET DOSE AND LOCK Position the ring-stop so that the side nearest the barrel is in line with the graduation mark (black line), and the required number of dogs shows between the ring-stop and the barrel. Lock the ring-stop by turning it away from you. Before dosing make sure that the ring-stop is locked. |
|
|
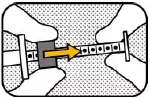
9. PULL CAP (TIGHT) Pull the cap strongly while holding the barrel. Note the cap is very tight (pull, do not twist). Save the cap for replacement. |
|
|
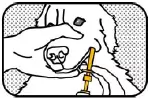
10. DOSE INTO CHEEK Place the oral syringe tip between the dog's cheek and gum and press the plunger until the ring-stop causes the plunger to stop. IMPORTANT: The gel should not be swallowed. If the gel is swallowed, it may not be effective. |
|
|
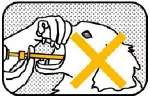
NOT SWALLOWED |
|
|
11. BACK TO PACKAGE Recap the oral syringe and return it to the outer package as the product is sensitive to light. Make sure that the carton is closed properly. Keep the package out of sight and reach of children at all times. Remove and discard gloves. |
Contra-indications, warnings, etc
If the oromucosal gel is swallowed it will become ineffective. Therefore feeding the dog or giving it treats within 15 minutes after administration of the gel should be avoided. In case the gel is swallowed the dog can be given another dose if necessary 2 hours after the previous dose. In extremely nervous, excited or agitated animals, the levels of endogenous catecholamines are often high. The pharmacological response elicited by alpha-2 agonists (e.g. dexmedetomidine) in such animals may be reduced. The safety of administering dexmedetomidine to puppies younger than 16 weeks and dogs over 17 years of age has not been studied. The safety of Sileo has not been established during pregnancy and lactation in the target species. Therefore, the use of the product during pregnancy and lactation is not recommended. Do not use in dogs with severe cardiovascular disorders. Do not use in dogs with severe systemic disease (graded as ASA III-IV) e.g. end stage renal or liver failure. Do not use in case of hypersensitivity to the active substance or to any of the excipients. Do not use in dogs obviously sedated from previous dosing. The use of other central nervous system depressants is expected to potentiate the effects of dexmedetomidine and therefore an appropriate dose adjustment should be made. Due to peripheral vasoconstriction, transient paleness of mucous membranes at the application site may be observed. Other commonly observed adverse events in clinical trials were sedation, emesis and urinary incontinence. Uncommon adverse reactions in clinical trials were anxiety, periorbital oedema, drowsiness and signs of gastroenteritis. The frequency of adverse reactions is defined using the following convention: - very common (more than 1 in 10 animals displaying adverse reactions during the course of one treatment) - common (more than 1 but less than 10 animals in 100 animals) - uncommon (more than 1 but less than 10 animals in 1,000 animals) - rare (more than 1 but less than 10 animals in 10,000 animals) - very rare (less than 1 animal in 10,000 animals, including isolated reports). Signs of sedation may occur when the dose is exceeded. The level and duration of sedation is dose dependent. If sedation occurs, the dog should be kept warm. Reduced heart rate may be seen after administration of higher than recommended doses of Sileo gel. Blood pressure decreases slightly below normal levels. Respiration rate can occasionally decrease. Higher than recommended doses of Sileo gel may also induce a number of other alpha-2 adrenoceptor mediated effects, which include mydriasis, depression of motor and secretory functions of the gastrointestinal tract, temporary AV-blocks, diuresis and hyperglycaemia. A slight decrease in body temperature may be observed. The effects of dexmedetomidine can be eliminated using a specific antidote, atipamezole (alpha-2 adrenoceptor antagonist). In case of overdose, the appropriate dose of atipamezole calculated in micrograms is 3 times (3X) the dose of administered dexmedetomidine hydrochloride in Sileo gel. Atipamezole (at the concentration of 5 mg/ml) dose in millilitres is one sixteenth (1/16th) of the dose volume of Sileo gel.
User warnings: In case of accidental ingestion or prolonged mucosal contact, seek medical advice immediately and show the package leaflet or the label to the physician. Do not drive as sedation and changes in blood pressure may occur. Avoid skin, eye or mucosal contact. Wear impermeable disposable gloves when handling the veterinary medicinal product. In case of skin contact wash the exposed skin immediately after exposure with large amounts of water and remove contaminated clothes. In case of eye or oromucosal contact, rinse abundantly with fresh water. If symptoms occur, seek the advice of a physician. People with known hypersensitivity to dexmedetomidine or any of the excipients should avoid contact with the veterinary medicinal product. Pregnant women should avoid contact with the product. Uterine contractions and decreased foetal blood pressure may occur after systemic exposure to dexmedetomidine.
Advice to the Doctor: Dexmedetomidine, the active ingredient of Sileo is an alpha-2 adrenoceptor agonist. Symptoms after absorption may involve clinical effects including dose-dependent sedation, respiratory depression, bradycardia, hypotension, a dry mouth, and hyperglycaemia. Ventricular arrhythmias have also been reported. Since effects are dose dependent they are more pronounced in small children than adults. Respiratory and haemodynamic symptoms should be treated symptomatically. The specific alpha-2 adrenoceptor antagonist, atipamezole, which is approved for use in animals, has been used in humans but only experimentally to antagonize dexmedetomidine-induced effects.
Pharmaceutical precautions
Keep the oral syringe in the carton in order to protect from light. Shelf life after first opening the immediate packaging (removal of the cap): 2 weeks. Any unused veterinary medicinal product or waste materials derived from such veterinary medicinal product should be disposed of in accordance with local requirements. Keep out of the sight and reach of children. For animal treatment only.
Legal Category: POM-V
To find details on all veterinary medicinal products currently authorised in the UK, please see the VMD's Product Information Database.
IMPORTANT LEGAL INFORMATION: this is a Veterinary Prescription Only Medicine (POM-V), which can only be prescribed by a veterinary surgeon, but may be dispensed by another veterinary surgeon or pharmacist.
DESCRIPTION
This product is sourced in the United Kingdom and is intended for use in the United Kindgom only.
SILEO (dexmedetomidine oromucosal gel) is an oromucosal gel formulation of dexmedetomidine hydrochloride provided in a 3 mL syringe at a concentration of 0.1 mg/mL. Using the syringe, SILEO is easily administered between the cheek and gum, allowing for transmucosal absorption. Because SILEO is formulated to provide a low dose of dexmedetomidine, the dog remains calm, yet fully functional, and is able to interact normally with the family.
Sileo® 0.1 mg/ml oromucosal gel for dogs
Species: Dogs Therapeutic indication: Pharmaceuticals: Neurological preparations: Others Active ingredient: Dexmedetomidine Product:Sileo® 0.1 mg/ml oromucosal gel for dogs Product index: Sileo
Presentation
Sileo is a translucent, green oromucosal gel, with each ml containing 0.1 mg dexmedetomidine hydrochloride (equivalent to 0.09 mg dexmedetomidine).
Uses
Indicated for the alleviation of acute anxiety and fear associated with noise in dogs.
Dosage and administration
Oromucosal use. The product should be administered onto the oral mucosa between the dog’s cheek and gum at a dose of 125 micrograms/m2. The Sileo oral syringe is capable of delivering the product in 0.25 ml increments. Each increment is shown as one dot on the plunger. The dosing table provides the number of dots to be administered corresponding to the dog’s bodyweight.  The following dosing table provides the dose volume (in dots) to be administered for the corresponding bodyweight. If the dose for the dog is more than 6 dots (1.5 ml), half of the dose should be administered to the oral mucosa on one side of the dog’s mouth and the other half of the dose onto the other side. Do not exceed the recommended dose.
The following dosing table provides the dose volume (in dots) to be administered for the corresponding bodyweight. If the dose for the dog is more than 6 dots (1.5 ml), half of the dose should be administered to the oral mucosa on one side of the dog’s mouth and the other half of the dose onto the other side. Do not exceed the recommended dose.
|
Bodyweight of dog (kg) |
Number of dots |
|
2.0 - 5.5 |
1 ° |
|
5.6 - 12 |
2 ° ° |
|
12.1 - 20 |
3 ° ° ° |
|
20.1 - 29 |
4 ° ° ° ° |
|
29.1 - 39 |
5 ° ° ° ° ° |
|
39.1 - 50 |
6 ° ° ° ° ° ° |
|
50.1 - 62.5 |
7 ° ° ° ° ° ° ° |
|
62.6 - 75.5 |
8 ° ° ° ° ° ° ° ° |
|
75.6 - 89 |
9 ° ° ° ° ° ° ° ° ° |
|
89.1 - 100 |
10 ° ° ° ° ° ° ° ° ° ° |
The first dose should be given as soon as the dog shows the first signs of anxiety, or when the owner detects a typical stimulus (e.g. sound of fireworks or thunder) for eliciting anxiety or fear in the respective dog. Typical signs of anxiety and fear are panting, trembling, pacing (frequent change of place, running around, restlessness), seeking people (clinging, hiding behind, pawing, following), hiding (under furniture, in dark rooms), trying to escape, freezing (absence of movements), refusing to eat food or treats, inappropriate urination, inappropriate defecation, salivation, etc. If the fear eliciting event continues and the dog shows signs of anxiety and fear again, re-dosing can be done when 2 hours has passed from the previous dose. The product can be dosed up to 5 times during each event.
Instructions for dosing the gel:
Dosing should be performed by an adult.
New oral syringe set up before first dosing:
|
1. WEAR GLOVES Wear impermeable disposable gloves when handling the veterinary medicinal product and handling the oral gel. |
|
|
2. HOLD PLUNGER Hold the oral syringe so that you can see the dot markings on the oral syringe plunger. Hold the plunger with your left hand. |
|
|
3. UNLOCK Hold the plunger with your left hand and unlock the green ring-stop by turning it towards you until it is able to slide freely. |
|
|
4. MOVE RING Move the ring-stop to the opposite end of the plunger. |
|
|
5. LOCK Hold the plunger with your right hand and lock the ring-stop by turning it away from you. |
Dose selection and dosing:
|
6. UNLOCK Hold the plunger with your right hand and unlock the ring-stop by turning it towards you. Do not pull the plunger! |
|
|
7. MOVE RING Move the ring-stop towards the other end of the plunger for choosing the correct dose based on your veterinarian's prescription. |
|
|
8. SET DOSE AND LOCK Position the ring-stop so that the side nearest the barrel is in line with the graduation mark (black line), and the required number of dogs shows between the ring-stop and the barrel. Lock the ring-stop by turning it away from you. Before dosing make sure that the ring-stop is locked. |
|
|
9. PULL CAP (TIGHT) Pull the cap strongly while holding the barrel. Note the cap is very tight (pull, do not twist). Save the cap for replacement. |
|
|
10. DOSE INTO CHEEK Place the oral syringe tip between the dog's cheek and gum and press the plunger until the ring-stop causes the plunger to stop. IMPORTANT: The gel should not be swallowed. If the gel is swallowed, it may not be effective. |
|
|
NOT SWALLOWED |
|
|
11. BACK TO PACKAGE Recap the oral syringe and return it to the outer package as the product is sensitive to light. Make sure that the carton is closed properly. Keep the package out of sight and reach of children at all times. Remove and discard gloves. |
Contra-indications, warnings, etc
If the oromucosal gel is swallowed it will become ineffective. Therefore feeding the dog or giving it treats within 15 minutes after administration of the gel should be avoided. In case the gel is swallowed the dog can be given another dose if necessary 2 hours after the previous dose. In extremely nervous, excited or agitated animals, the levels of endogenous catecholamines are often high. The pharmacological response elicited by alpha-2 agonists (e.g. dexmedetomidine) in such animals may be reduced. The safety of administering dexmedetomidine to puppies younger than 16 weeks and dogs over 17 years of age has not been studied. The safety of Sileo has not been established during pregnancy and lactation in the target species. Therefore, the use of the product during pregnancy and lactation is not recommended. Do not use in dogs with severe cardiovascular disorders. Do not use in dogs with severe systemic disease (graded as ASA III-IV) e.g. end stage renal or liver failure. Do not use in case of hypersensitivity to the active substance or to any of the excipients. Do not use in dogs obviously sedated from previous dosing. The use of other central nervous system depressants is expected to potentiate the effects of dexmedetomidine and therefore an appropriate dose adjustment should be made. Due to peripheral vasoconstriction, transient paleness of mucous membranes at the application site may be observed. Other commonly observed adverse events in clinical trials were sedation, emesis and urinary incontinence. Uncommon adverse reactions in clinical trials were anxiety, periorbital oedema, drowsiness and signs of gastroenteritis. The frequency of adverse reactions is defined using the following convention: - very common (more than 1 in 10 animals displaying adverse reactions during the course of one treatment) - common (more than 1 but less than 10 animals in 100 animals) - uncommon (more than 1 but less than 10 animals in 1,000 animals) - rare (more than 1 but less than 10 animals in 10,000 animals) - very rare (less than 1 animal in 10,000 animals, including isolated reports). Signs of sedation may occur when the dose is exceeded. The level and duration of sedation is dose dependent. If sedation occurs, the dog should be kept warm. Reduced heart rate may be seen after administration of higher than recommended doses of Sileo gel. Blood pressure decreases slightly below normal levels. Respiration rate can occasionally decrease. Higher than recommended doses of Sileo gel may also induce a number of other alpha-2 adrenoceptor mediated effects, which include mydriasis, depression of motor and secretory functions of the gastrointestinal tract, temporary AV-blocks, diuresis and hyperglycaemia. A slight decrease in body temperature may be observed. The effects of dexmedetomidine can be eliminated using a specific antidote, atipamezole (alpha-2 adrenoceptor antagonist). In case of overdose, the appropriate dose of atipamezole calculated in micrograms is 3 times (3X) the dose of administered dexmedetomidine hydrochloride in Sileo gel. Atipamezole (at the concentration of 5 mg/ml) dose in millilitres is one sixteenth (1/16th) of the dose volume of Sileo gel.
User warnings: In case of accidental ingestion or prolonged mucosal contact, seek medical advice immediately and show the package leaflet or the label to the physician. Do not drive as sedation and changes in blood pressure may occur. Avoid skin, eye or mucosal contact. Wear impermeable disposable gloves when handling the veterinary medicinal product. In case of skin contact wash the exposed skin immediately after exposure with large amounts of water and remove contaminated clothes. In case of eye or oromucosal contact, rinse abundantly with fresh water. If symptoms occur, seek the advice of a physician. People with known hypersensitivity to dexmedetomidine or any of the excipients should avoid contact with the veterinary medicinal product. Pregnant women should avoid contact with the product. Uterine contractions and decreased foetal blood pressure may occur after systemic exposure to dexmedetomidine.
Advice to the Doctor: Dexmedetomidine, the active ingredient of Sileo is an alpha-2 adrenoceptor agonist. Symptoms after absorption may involve clinical effects including dose-dependent sedation, respiratory depression, bradycardia, hypotension, a dry mouth, and hyperglycaemia. Ventricular arrhythmias have also been reported. Since effects are dose dependent they are more pronounced in small children than adults. Respiratory and haemodynamic symptoms should be treated symptomatically. The specific alpha-2 adrenoceptor antagonist, atipamezole, which is approved for use in animals, has been used in humans but only experimentally to antagonize dexmedetomidine-induced effects.
Pharmaceutical precautions
Keep the oral syringe in the carton in order to protect from light. Shelf life after first opening the immediate packaging (removal of the cap): 2 weeks. Any unused veterinary medicinal product or waste materials derived from such veterinary medicinal product should be disposed of in accordance with local requirements. Keep out of the sight and reach of children. For animal treatment only.
Legal Category: POM-V
To find details on all veterinary medicinal products currently authorised in the UK, please see the VMD's Product Information Database.
IMPORTANT LEGAL INFORMATION: this is a Veterinary Prescription Only Medicine (POM-V), which can only be prescribed by a veterinary surgeon, but may be dispensed by another veterinary surgeon or pharmacist.